Thermal relaxation of lithium dendrites†
Abstract
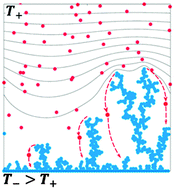
The average lengths ![[small lambda, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cc.gif) of lithium dendrites produced by charging symmetric Li0 batteries at various temperatures are matched by Monte Carlo computations dealing both with Li+ transport in the electrolyte and thermal relaxation of Li0 electrodeposits. We found that experimental
of lithium dendrites produced by charging symmetric Li0 batteries at various temperatures are matched by Monte Carlo computations dealing both with Li+ transport in the electrolyte and thermal relaxation of Li0 electrodeposits. We found that experimental ![[small lambda, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cc.gif) (T) variations cannot be solely accounted by the temperature dependence of Li+ mobility in the solvent but require the involvement of competitive Li-atom transport from metastable dendrite tips to smoother domains over ΔE‡R ∼ 20 kJ mol−1 barriers. A transition state theory analysis of Li-atom diffusion in solids yields a negative entropy of activation for the relaxation process: ΔS‡R ≈ −46 J mol−1 K−1 that is consistent with the transformation of amorphous into crystalline Li0 electrodeposits. Significantly, our ΔE‡R ∼ 20 kJ mol−1 value compares favorably with the activation barriers recently derived from DFT calculations for self-diffusion on Li0(001) and (111) crystal surfaces. Our findings suggest a key role for the mobility of interfacial Li-atoms in determining the morphology of dendrites at temperatures above the onset of surface reconstruction: TSR ≈ 0.65 TMB (TMB = 453 K: the melting point of bulk Li0).
(T) variations cannot be solely accounted by the temperature dependence of Li+ mobility in the solvent but require the involvement of competitive Li-atom transport from metastable dendrite tips to smoother domains over ΔE‡R ∼ 20 kJ mol−1 barriers. A transition state theory analysis of Li-atom diffusion in solids yields a negative entropy of activation for the relaxation process: ΔS‡R ≈ −46 J mol−1 K−1 that is consistent with the transformation of amorphous into crystalline Li0 electrodeposits. Significantly, our ΔE‡R ∼ 20 kJ mol−1 value compares favorably with the activation barriers recently derived from DFT calculations for self-diffusion on Li0(001) and (111) crystal surfaces. Our findings suggest a key role for the mobility of interfacial Li-atoms in determining the morphology of dendrites at temperatures above the onset of surface reconstruction: TSR ≈ 0.65 TMB (TMB = 453 K: the melting point of bulk Li0).


 Please wait while we load your content...
Please wait while we load your content...