New meso-substituted corroles possessing pentafluorophenyl groups – synthesis and spectroscopic characterization†
Abstract
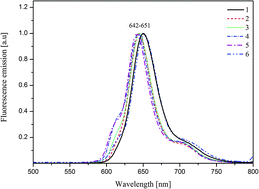
The investigation presented in this paper deals with new free-base corroles substituted with different peripheral groups. These aromatic macrocycles were efficiently synthesized by a [2+1] approach from dipyrromethanes. Moreover, the basic spectroscopic studies of the dyes in chloroform were conducted, and the UV-Vis absorption, fluorescence and ESR parameters were estimated. The experimental data were supported by quantum chemical calculations. The presence of monomeric dye structures is concentration independent (10−6–10−4 M), as expected for dyes in a solvent of low polarity, and rules out aggregate formation of corroles dissolved in chloroform. The excitation emission and fluorescence life-time values confirm the monomeric structure of the corroles. The spectra were compared with the time-dependent density functional theory (TD-DFT) results for the HOMO–LUMO states. The ESR examinations strongly show that for any type of studied fluorine corrole an unpaired electron is localized on the corrole macroring but not on the substituents both before and after light illumination. Laser illumination creates additional radicals, however with different effectiveness depending on the sample.

 Please wait while we load your content...
Please wait while we load your content...