Synthesis and photophysical properties of a “face-to-face” stacked tetracene dimer†
Abstract
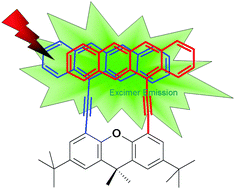
A covalently linked tetracene dimer has been prepared and its molecular structure is characterized by 1H NMR and MALDI-TOF mass spectroscopy, and elemental analysis. The minimized molecular structure reveals that the tetracene subunits in a dimer adopt a “face-to-face” stacked configuration. Its absorption spectrum differs significantly from that of the monomeric counterpart in solution, suggesting the presence of strong interactions between the two tetracene subunits. In solution, the fluorescence spectrum is dominated by a band at around 535 nm, due to an oxidative impurity. In the longer wavelength range, a short-lived lower energy emission can be identified as the intrinsic emission of the dimer. In a polystyrene matrix or at low temperatures, the lifetime of the lower energy emission lengthens and it becomes more prominent. We suggest that the interactions between the two tetracene subunits produce a short-lived, lower energy “excimer-like” state. The fluorescence decays show no observable dependence on an applied magnetic field, and no obvious evidence of significant singlet fission is found in this dimer. This research suggests that even though there are strong electronic interactions between the tetracene subunits in the dimer, singlet fission cannot be achieved efficiently, probably because the formation of “excimer-like” states competes effectively with singlet fission.

 Please wait while we load your content...
Please wait while we load your content...