A femtosecond mid-infrared study of the dynamics of water in aqueous sugar solutions†
Abstract
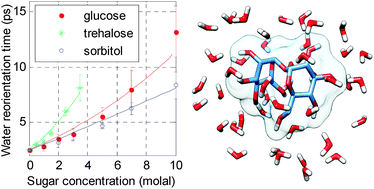
We study the effect of the sugars glucose, trehalose and sorbitol on the reorientation dynamics of water molecules, using polarization-resolved femtosecond infrared spectroscopy. We find that at all sugar concentrations the water dynamics can be described by a single reorientation time constant. With increasing carbohydrate concentration, the water reorientation time constant increases from 2.5 picoseconds to a value of about 15 picoseconds. The slowing down of the water dynamics is strongest for trehalose, followed by glucose and sorbitol.
- This article is part of the themed collection: Bunsentagung 2015: Solvation Science

 Please wait while we load your content...
Please wait while we load your content...