Long-range ion–water and ion–ion interactions in aqueous solutions
Abstract
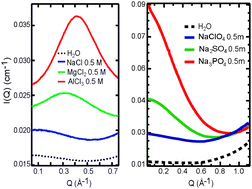
Using small-angle X-ray scattering (SAXS), we obtained direct experimental evidence on the structure of hydrated polyatomic anions, with hydration effects starkly different from those of cations (J. Chem. Phys., 2011, 134, 064513). We propose that the size and charge density of the naked ions do not sufficiently account for the differences in the SAXS curves. For cations, the ion–ion contribution gives a prominent first-order diffraction peak, whereas for anions, the low-Q enhancement in the SAXS curves indicates density inhomogeneities as a result of ion–water interactions.
- This article is part of the themed collection: Bunsentagung 2015: Solvation Science

 Please wait while we load your content...
Please wait while we load your content...