Salt-specific effects observed in calorimetric studies of alkali and tetraalkylammonium salt solutions of poly(thiophen-3-ylacetic acid)†
Abstract
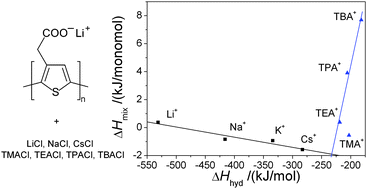
The enthalpies of dilution ΔHdil of aqueous solutions of a conjugated polyelectrolyte, poly(thiophen-3-ylacetic acid), neutralized by lithium, sodium, cesium, tetramethyl-, tetraethyl-, tetrapropyl-, and tetrabutylammonium hydroxides, were determined in the concentration range from cp = 2 × 10−3 to 1 × 10−1 monomol dm−3 and for T = 278.15, 298.15, and 318.15 K. At low concentrations the dilution of the alkali PTAA salts yields an endothermic effect, which is in part a consequence of the hydrolysis. An exception is PTALi at 278.15 K, where ΔHdil < 0. In the case of tetraalkylammonium salts the enthalpies of dilution increase in the order TBA < TPA < TEA < TMA. Only the TBA salt of PTAA yields an exothermic effect upon dilution in the whole temperature range. In the second part of the study we measured the enthalpies of mixing, ΔHmix, of various salts of poly(thiophen-3-ylacetic acid) with LiCl, NaCl, KCl, and CsCl solutions in water. When lithium salt of PTAA is mixed with LiCl ΔHmix is positive. For mixing experiments with other alkali chlorides the effect is exothermic. In addition, the enthalpies of mixing of PTALi with tetramethyl-, tetraethyl-, tetrapropyl-, and tetrabutylammonium chloride were measured at T = 278.15 K, 298.15 K, and 318.15 K. Popular polyelectrolyte theories, such as Manning's limiting law, predict for the heat to be released upon dilution, and consumed upon mixing; the agreement between this purely electrostatic theory and experiments is at best qualitative. The ΔHmix values are correlated with the enthalpies of hydration of the cations of the low molecular mass salts added to the solution.

![[r with combining breve]](https://www.rsc.org/images/entities/char_0072_0306.gif) í
Vohlídal
í
Vohlídal Please wait while we load your content...
Please wait while we load your content...