Determination of oxygen adsorption–desorption rates and diffusion rate coefficients in perovskites at different oxygen partial pressures by a microkinetic approach†
Abstract
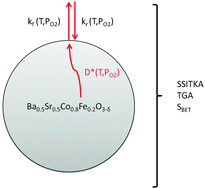
A novel, powerful method based on a microkinetic approach is described for the estimation of the oxygen transport parameters of mixed electronic conducting materials (MIECs). This method is validated on the perovskite La0.6Sr0.4Co0.2Fe0.8O3−δ and has been applied on Ba0.5Sr0.5Co0.8Fe0.2O3−δ. This approach is original and relevant in that the surface kinetic rate constants are measured using a sample in powder form. In contrast to methods previously used, such as isotope exchange depth profiling (IEDP) and electrical conductivity relaxation (ECR), which determine the global exchange kinetic parameter, our microkinetic modelling approach allows the estimation of the forward and reverse kinetic rates accounting for the oxygen vacancy concentration. Also, the self-diffusion rate coefficient has been estimated at different oxygen partial pressures. This microkinetic approach, which combines SSITKA (steady-state isotopic transient kinetic analysis) and thermogravimetric measurements at controlled oxygen partial pressure, has the potential to significantly accelerate the characterization of oxygen transport in perovskites and related materials in the future. In this study, the kinetic parameters were measured in a temperature window between 873 K and 1173 K, and at two oxygen pressure conditions (21 kPa and 1 kPa) that are appropriate for simulating the semi-permeability of oxygen in a membrane in a process of oxygen separation from air.

 Please wait while we load your content...
Please wait while we load your content...