Surface redox chemistry and mechanochemistry of insulating polystyrene nanospheres†
Abstract
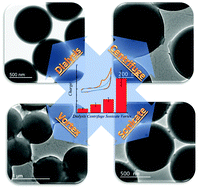
Cyclic voltammetry (CV) of polystyrene nanospheres was carried out after immobilisation onto boron-doped diamond electrodes. Although the polystyrene is insulating, a voltammetric response was obtained. This was attributed to the high surface area of the nanospheres, allowing the redox chemistry of the polystyrene surface to be probed despite the non-conducting nature of the bulk. The polystyrene redox response was found to be strongly dependent on prior mechanical agitation. Centrifuged, sonicated and vortexed polystyrene nanospheres all exhibited significantly higher oxidation currents than the non-agitated polystyrene. Mechanical treatment by sonication and centrifugation was found to bring about changes to surface chemistry of the polystyrene spheres, in particular the introduction of oxygen functionalities. For these samples the CV response is attributed to the presence of surface phenol functionalities. On the non-agitated and vortex treated polystyrene surfaces X-ray photoelectron spectroscopy revealed an absence of oxygen functionalities that could explain the redox response. Repetition of the CV experiment in the presence of a solution spin trap suggests that radical species play a role in the observed response. For the vortexed sample the increased oxidation currents were attributed to significant surface roughening and deformation, as revealed by Transmission Electron Microscopy.

 Please wait while we load your content...
Please wait while we load your content...