Encapsulation of an f-block metal atom/ion to enhance the stability of C20 with the Ih symmetry
Abstract
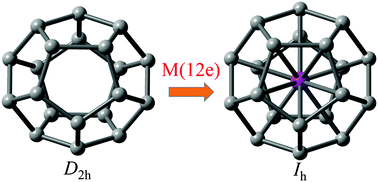
Based on the density functional theory, the geometric and electronic structures, chemical stability, and bonding properties of the endohedral metallofullerenes, M@C20 (M = Eu3−, Am3−, Gd2−, Cm2−, Tb−, Bk−, Dy, Cf, Ho+, Es+, Er2+, Fm2+, Tm3+, Md3+, Yb4+, No4+, Lu5+, and Lr5+), were investigated. Through encapsulation of an f-block metal atom/ion with 12 valence electrons, the bare C20 cage with the D2h point group could be stabilized to a highly symmetrical Ih structure. The calculated values of HOMO–LUMO energy gaps using the B3lYP and BHHLYP functionals ranged from 2.22 to 5.39 eV and from 3.89 to 7.95 eV, respectively. The stability of these metal-encapsulated clusters can be attributed to the 32-electron rule, where the central metal atom's orbitals strongly participated in the t2u, gu, t1u, hg, and ag valence molecular orbitals.

 Please wait while we load your content...
Please wait while we load your content...