New Ni(ii) 1,2-bis(diphenylphosphino)ethane dithiolates: crystallographic, computational and Hirshfeld surface analyses†
Abstract
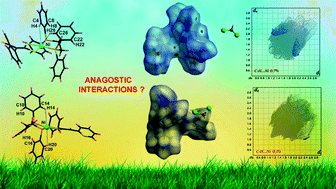
Three new nickel(II) 1,2-bis(diphenylphosphino)ethane dithiolate complexes viz. [Ni(dppe)(benzylcyanidedithiolate)] (1), [Ni(dppe)(2-cyanobenzylcyanidedithiolate)] (2) and [Ni(dppe)(pyridine-2-cyanidedithiolate)] (3) have been synthesized and characterized spectroscopically and by single-crystal X-ray analysis. The X-ray analyses of 2 and 3 reveal a distorted square planar geometry around Ni(II) satisfied by the two sulfur atoms of the dithiolate and two phosphorus atoms of the dppe ligands. Both 2 and 3 display intermolecular C–N⋯H, π⋯π and C–H⋯π and C–S⋯H interactions which generate a supramolecular framework. Additionally, both 2 and 3 show intramolecular C–H⋯Ni anagostic interactions. These interactions have been addressed by ab initio, AIM and Hirshfeld surface analyses. The anagostic interactions are verified using bond order and natural charge calculations which indicated depletion of electron density on the ortho-hydrogen atoms undergoing the anagostic interactions. All three compounds exhibit photoluminescence in dichloromethane solution as well as in the solid state. The solid-state emissions are red-shifted in comparison to the solution-phase emissions which may be attributed to the existence of intermolecular interactions in the solid state.

 Please wait while we load your content...
Please wait while we load your content...