Structural diversities in Cu(i) and Ag(i) sulfonate coordination polymers and their anion exchange properties†
Abstract
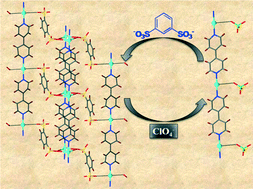
Two new Cu(I) compounds, namely [Cu2(bds)(bpy)2]·2H2O (1) and [Cu4(bds)2(azpy)4]·6H2O (3) (where bds = benzene-1,3-disulfonate, bpy = 4,4′-bipyridine and azpy = 4,4′-azopyridine), and four Ag(I) compounds, namely [Ag2(bds)(bpy)2]·2H2O (2), [Ag2(bds)(azpy)2]·4H2O (4), [Ag(bds)1/2(bpe)]·3H2O (5), and [Ag4(bds)2(tmdp)4]·9H2O (6) (where bpe = 1,2-di(4-pyridyl)ethylene and tmdp = 4,4′-trimethylenedipyridine), have been synthesized, and their structures were determined and characterized by elemental analysis, IR, UV-vis and thermal studies. The structure of the compounds changed from 1D (1 and 2) to 2D (3–5) and interpenetrated 3D (6). In the case of 5, a solid-state [2 + 2] photochemical cycloaddition reaction has been performed. Compound 2 exhibits a reversible anion exchange for perchlorate and permanganate, whereas the other compounds (1, 3–6) exhibit an irreversible anion exchange behaviour for perchlorate. Catalytic studies on 2 indicate Lewis acidity.

 Please wait while we load your content...
Please wait while we load your content...