In situ synchrotron XRD investigation of the dehydration and high temperature carbonation of Ca(OH)2†
Abstract
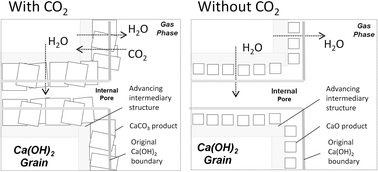
The dehydration and high temperature carbonation reactions of Ca(OH)2 are an essential part of a number of existing schemes aiming to apply the thermochemical CaO–CaCO3 cycle (‘Ca Looping’) in emerging energy industries such as CO2 capture, gasification and thermal energy storage. However, the underlying chemical mechanisms are poorly understood and the reaction kinetics exhibit a number of strange features such as the increased thermal stability of Ca(OH)2, dubbed “superheating”, observed in the presence of CO2. In this work, in situ X-ray diffraction (XRD) measurements using a synchrotron source are coupled with full pattern fitting analysis to provide high-resolution and high-accuracy data on the evolution of Ca(OH)2 crystalline structural parameters during the dehydration and carbonation reactions. This method has allowed new observations to be made that provide insight into the studied reaction mechanisms and disprove existing hypotheses as to the origin of superheating. A new hypothesis, involving the formation of an intermediate and permeable Ca(OH)2 structure was formulated which can explain the growing body of observations in this area.

 Please wait while we load your content...
Please wait while we load your content...