Synthesis, structure and magnetic properties of hydroxychlorides A3Cu3(OH)Cl8 (A = Cs, Rb) with isolated tricopper†
Abstract
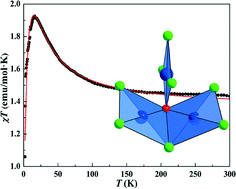
Two copper hydroxychlorides, A3Cu3(OH)Cl8 (A = Cs, Rb), were synthesized by a hydrothermal method, which crystallize in the space group P21/c. All copper atoms are coordinated with three Cl atoms and one OH group forming quite distorted squares of CuCl3(OH), which further share one edge and one corner to form an isolated triangle unit of [Cu3(OH)Cl8]3− with μ2-Cl and μ3-OH cores. Interestingly, the two isostructural compounds exhibit antiferromagnetic ordering at low temperature and the dominant exchange coupling in the isolated triangle is found to be of ferromagnetic type, ruling out geometrical frustration effects. The origin of such a ferromagnetic interaction is suggested to arise likely from the folded [Cu(2)Cu(3)(OH)Cl5]2− dimers with a Cu(2)–Cl(5)–Cu(3) route.

 Please wait while we load your content...
Please wait while we load your content...