Hydrothermal synthesis and photoluminescence properties of Ca9Eu(PO4)7 nanophosphors
Abstract
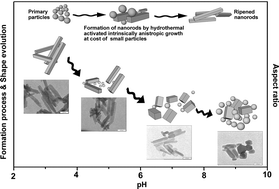
The red-emitting Ca9Eu(PO4)7 nanophosphors have been successfully synthesized by a facile hydrothermal method. X-ray diffraction (XRD), photoluminescence (PL) spectroscopy, GSAS structural refinement and scanning electron microscopy (SEM) were used to characterize the materials. Rietveld refinement of powder X-ray diffraction patterns of Ca9Eu(PO4)7 demonstrated that the structure of Ca9Eu(PO4)7 consisted of [PO4] tetrahedra. Ca/Eu atoms filled the space between the tetrahedral groups with Eu3+ ions distributed randomly on three individual Ca crystallographic sites. The major red emission peak located at 616 nm under 397 nm excitation attributed to the electric dipole transition 5D0 → 7F2 of Eu3+ was a parity forbidden f–f configuration transition, which would only occur when Eu3+ occupied lattice sites without inversion symmetry. Introducing Gd, La, and Y ions into samples made luminescence properties of Ca9Eu1−xLnx(PO4)7 (Ln = Gd, La, Y) phosphors decrease. pH value plays a key role in the formation of Ca9Eu(PO4)7 nanophosphor morphology. With increasing pH in the initial reaction solution, the morphology of samples changed from rod to sphere and their aspect ratio also decreased. Different morphologies have different photoluminescence properties, and samples prepared at pH 7 manifested better photoluminescence properties relative to other samples. This novel red-emitting phosphor could be applied in white light-emitting diodes.

 Please wait while we load your content...
Please wait while we load your content...