Large-scale one pot synthesis of metal oxide nanoparticles by decomposition of metal carbonates or nitrates†
Abstract
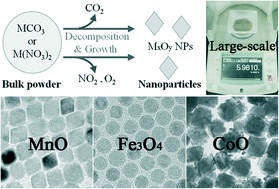
This study identifies bulk metal carbonates and nitrates as cost-effective starting materials for a large-scale, one-pot synthesis of monodisperse metal oxide nanoparticles of MnO, Fe3O4, and CoO phases. The thermal decomposition of bulk metal carbonates or nitrates in hot fatty acid forms monodisperse metal oxide nanoparticles on a large scale. The release of carbon monoxide and nitrogen dioxide gas molecules from these precursors would in situ form molecular metal-oxo species, lightly stabilized by complexation with fatty acid, which would in turn undergo subsequent agglomeration to form metal oxide nanoparticles. The particle size and shape could be controlled simply by varying the reaction conditions, namely the amount and nature of added alkylamine. This general and convenient synthetic methodology would be greatly beneficial to the practical application of thermally synthesized metal oxide nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...