Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: the role of hydrofluoric acid in crystallization†
Abstract
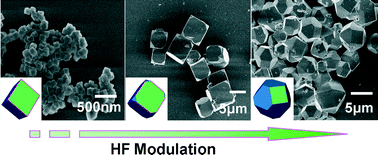
UiO-66 with crystal size ranging from hundreds of nanometers to a few micrometers and with cubic and cuboctahedral morphologies were synthesized. Crystal size and morphology varied with the additive amount of hydrofluoric acid and the concentration of reactants (ZrCl4 and H2BDC) during solvothermal synthesis. According to energy dispersive spectrometry (EDS) and 19F MAS NMR measurements, the fluorine ions directly bonded to Zr in the SBUs (secondary building units) in the MOF framework due to their strongest electronegativity. The bonding of the fluorine ions and Zr not only compensated for the charge imbalance of the framework caused by missing linkers but also competed with the linkers to coordinate with the Zr metal centers, thereby controlling the processes of nucleation and growth of the UiO-66 crystals. The samples were further characterized by scanning electron microscopy (SEM), powder X-ray diffraction (XRD), Fourier transform-infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA) and Ar sorption isotherms, showing that the introduction of fluorine enhanced the thermostability and porosity of UiO-66.

 Please wait while we load your content...
Please wait while we load your content...