Controlled formation of barium fluoride nanocrystals by electric-assisted phase separation and precipitation
Abstract
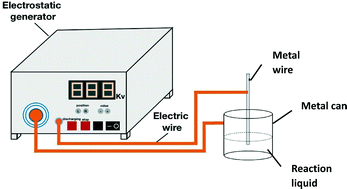
This work demonstrated that barium fluoride (BaF2) nanocrystals can be controllably formed by an electric-assisted phase separation and precipitation method, EAPSP, in a water/ethanol mixture. Experimentally, we employed an electrostatic generator to provide various voltages to reduce the surface tension of a water/ethanol mixture to cause rapid phase separation after which BaF2 nanocrystals formed and precipitated under control. Results showed that the average diameter of the formed BaF2 nanocrystals was reduced with the voltage increase, e.g. the 0 V sample at about 70 nm in diameter, the 500 V sample at 50 nm in diameter and the 1000 V sample at 30 nm in diameter. The conductivity of these nanocrystals was found to increase with the decrease in size.

 Please wait while we load your content...
Please wait while we load your content...