Molecular simulation of oligo-glutamates in a calcium-rich aqueous solution: insights into peptide-induced polymorph selection†
Abstract
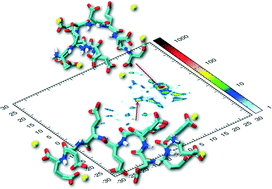
The presence of oligopeptides often has a drastic impact on the nucleation and crystal growth of calcium-containing minerals. Molecular simulation of complexes of peptides with Ca2+ ions can give mechanistic insight into how the pre-alignment of ions from solution by additives may steer the crystallization of calcium rich minerals into different polymorphs. We exploit advanced molecular modeling techniques to sample and analyze the complex multidimensional configurational space of aqueous solutions of oligo-glutamate and Ca2+ ions. We find that glutamate oligomers induce the formation of patterns in the associated Ca2+ ions in solution, which can serve as templates for the growth of different calcium oxalate pseudopolymorphs – depending on the peptide length, in excellent agreement with experimental observations. Glutamate decamers prestructure the Ca2+ ions in remarkable correspondence with typical distances in calcium oxalate dihydrate. These are distinct from those in calcium oxalate trihydrate which typically grows in the absence of peptide or in the presence of shorter glutamate oligomers such as pentamers.
- This article is part of the themed collection: Fundamentals of Nanocrystal Formation

 Please wait while we load your content...
Please wait while we load your content...