Coordination polymers constructed from a tripodal phosphoryl carboxylate ligand: synthesis, structures and physical properties†
Abstract
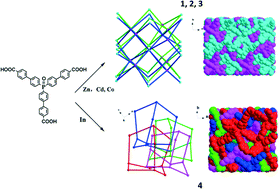
By employment of a new tripodal phosphoryl carboxylate ligand, tris(p-carboxyl-biphenyl)phosphine oxide (H3TBPPO), four novel coordination polymers, namely, Zn3(TBPPO)2 (1), Cd3(TBPPO)2 (2), Co3(TBPPO)2 (3) and In(TBPPO) (4), have been synthesized and characterized. Complexes 1, 2 and 3 are isostructural. Crystal structure analysis reveals that complexes 1–3 consist of trinuclear metal building units, which are further bridged by the tripodal phosphine oxide carboxylate ligands to give a (4,8)-connected 2-fold interpenetrating flu topological net with a point symbol of (412·612·84)(46)2, while complex 4 possesses a 3D 4-fold interpenetrating dia network with a point symbol of (66). Luminescence measurements indicate that complexes 1, 2 and 4 all show strong emission bands at around 378 nm in the solid state at room temperature. Furthermore, temperature-dependent magnetic susceptibility study shows that complex 3 exhibits antiferromagnetism properties.

 Please wait while we load your content...
Please wait while we load your content...