Influence of lower rim C-methyl group on crystal forms and metal complexation of resorcinarene bis-crown-5†
Abstract
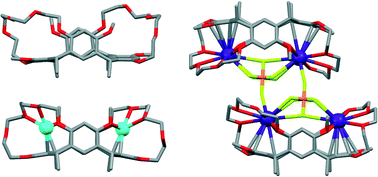
C-Methyl resorcinarene bis-crown-5 (1) with pendant methyl groups at the lower rim was prepared and crystallized in various solvent mixtures with and without selected metal salts. The crystal structures of two polymorphic forms of unsolvated 1 (1-I and 1-II), three solvates (acetonitrile, chloroform and dichloromethane–methanol), and three metal complexes with silver and cesium salts were obtained. The lower rim methyl groups and the block shape of the host promote crystal packing in brick-wall type assemblies, in which the binding cavities are efficiently filled by the crown bridges. Thus, solvents are found in the interstitial space or coordinated to the crown bridges on top of the cavity, whereas more strongly binding metal cations are able to occupy the cavity. The chloroform solvate proved to be a relatively labile crystal form, which transformed to unsolvated form (1-I) over time. Resorcinarene mono-crown-5 (2) was obtained as a minor product of the bridging reaction. Two solvate structures (acetonitrile and chloroform–water) of 2 are also reported, providing an example of the effect of the missing crown bridge on the solvate structures.

 Please wait while we load your content...
Please wait while we load your content...