Morphological variation of anatase TiO2 crystals via formation of titanium glycerolate precursors under microwave irradiation†
Abstract
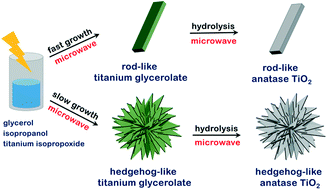
The morphologies of anatase TiO2 crystals have been varied facilely from rod-like structures to various hedgehog-like hierarchical structures via formation of titanium glycerolate precursors as sacrificial templates. The morphologies of the precursors have been readily controlled under microwave irradiation by adjusting the relative solvent volumes of isopropanol and glycerol. The simple hydrolysis of the as-prepared titanium glycerolate precursors for 10 min under microwave irradiation has yielded intrinsic morphology-retained anatase TiO2 nanostructures. The smooth surfaces of the precursors have been turned into rough surfaces of anatase TiO2 crystals during the hydrolysis, suggesting that our as-prepared anatase TiO2 nanostructures have high potential applications in diverse fields such as photocatalysis and in solar cells. It has been suggested that the variation of the relative volume fractions of isopropanol and glycerol having significantly different boiling points and viscosity values has changed the nucleation and growth kinetics so the morphologies as well as the sizes of titanium glycerolate precursors can be controlled.

 Please wait while we load your content...
Please wait while we load your content...