A family of coordination polymers assembled with a flexible hexacarboxylate ligand and auxiliary N-donor ligands: syntheses, structures, and physical properties†
Abstract
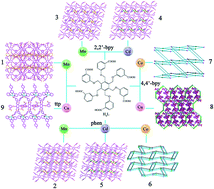
Nine new coordination polymers, namely, [Mn5(HL)2(H2O)2]·2C2H5OH·4H2O (1), Mn3(L)(phen)2 (2), Mn3(L)(2,2′-bpy)2 (3), Cd3(L)(2,2′-bpy)2 (4), Cd3(L)(phen)2 (5), Co2Na(HL)(phen)2·0.5C2H5OH·H2O (6), Co3(L)(4,4′-bpy) (7), Cu2(H2L)(4,4′-bpy)4·0.5H2O (8) and Cu2(H2L)(ttp)2·2C2H5OH·2H2O (9), have been synthesized under hydrothermal conditions (H6L = 1,2,3,4,5,6-hexakis(3-carboxyphenyloxymethylene)benzene, phen = 1,10-phenathroline, 2,2′-bpy = 2,2′-bipyridine, 4,4′-bpy = 4,4′-bipyridine and ttp = 2-(6-(pyridin-2-yl)-4-p-tolylpyridin-2-yl)pyridine). Compound 1 shows a 2D layer structure. Compounds 2–5 display similar 2D networks, which are further extended into a 3D supramolecular architecture by π–π interactions. Compound 6 exhibits a 3D binodal 4-connected framework with (42638)2 topology. Compound 7 furnishes a 3D (4,6)-connected framework with (4462)(446108) topology. Compound 8 reveals a 3D 4-connected framework with 66 topology. Compound 9 displays a 1D chain structure, which is further linked by hydrogen-bonding interactions to yield a 3D supramolecular architecture. Moreover, the diffuse reflectivity spectra of all the compounds, the solid state photoluminescence properties of compounds 4–5, and magnetic properties of compounds 1, 3, 6 and 7 were studied.

 Please wait while we load your content...
Please wait while we load your content...