Positional isomerism in triarylmethyl carbocation radical salts: positional isomeric effects, crystal structures and properties†
Abstract
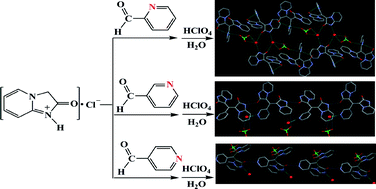
Through a simple and mild approach, we unprecedentedly synthesized three novel triarylmethyl carbocation radical salts, namely bisimidazo[1,2-a]pyridin-2-one-yl(2-pyridyl)methyl carbocation radical salt (1), bisimidazo[1,2-a]pyridin-2-one-yl(3-pyridyl)methyl carbocation radical salt (2), and bisimidazo[1,2-a]pyridin-2-one-yl(4-pyridyl)methyl carbocation radical salt (3). Single-crystal X-ray diffraction analyses reveal that 1, 2 and 3 are positional isomers with different one-dimensional (1D) chain structures. Through supramolecular interactions, the 1D chains of 1 are further assembled into a 1D double-stranded chain, then into a two-dimensional (2D) layer structure, and the 1D chains of 2 and 3 are also assembled into 2D layer structures. This work indicates that the positional isomeric effects of pyridyl groups in the three carbocation radical salts are significant in the construction of isomers, because 1, 2 and 3 were synthesized and crystallized under the same conditions. Furthermore, these positional isomers exhibit different magnetic susceptibility values at 300 K. 1 with 1D double-stranded chains consists of one repetitive unit having four carbocation radical moieties, giving a magnetic susceptibility value of 1.636 emu K Oe−1 mol−1 at 300 K. 2 with 1D chains consists of one repetitive unit with two carbocation radical moieties, giving a magnetic susceptibility value of 0.747 emu K Oe−1 mol−1 at 300 K. 3 exhibits a magnetic susceptibility value of 0.326 emu K Oe−1 mol−1 for one isolated spin of the carbocation radical. Compared with 2 and 3, 1 shows broad and stronger NIR absorption bands from 800 nm to 1951 nm, probably ascribed to its 1D double-stranded chain structure, antiparallel arrangements of 2-pyridinium rings in the 1D chains, and/or tetra-connecting hydrogen-bonding bridge.

 Please wait while we load your content...
Please wait while we load your content...