Polar and antiferroelectric behaviour of a hybrid crystal – piperazinium perchlorate†
Abstract
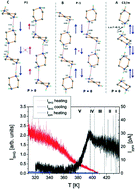
Monoprotonated piperazinium perchlorate, [NH2(CH2)4NH][ClO4], appeared to be a novel room temperature polar material (P1). Its acentric symmetry was confirmed by single-crystal X-ray diffraction, second harmonic generation (SHG) and pyroelectric measurements. Differential scanning calorimetry (DSC) measurements revealed a complex sequence of phase transitions above room temperature: I ↔ II at 433/422 K (heating–cooling), II ↔ III at 417/411 K, III ↔ IV at 403/395 K and IV ↔ V at 397 K (the lowest temperature phase transition recorded only upon heating). The characteristic feature of the structure of [NH2(CH2)4NH][ClO4] is the presence of two parallel cationic chains which are connected with each other by strong N–H⋯N hydrogen bonds. In phase V, these strongly polar non-equivalent chains contribute to spontaneous polarization. 1H NMR measurements disclosed the reorientational motions of the piperazinium ([NH2(CH2)NH]+) cations as well as the proton motion in the N–H⋯N hydrogen bonds along the piperazine chain. Over phase I, the overall motions of the ClO4− anions and reorientational motion of cations are postulated. The dielectric response, ε′(T), accompanying the PT I ↔ II indicates possible antiferroelectricity in phase II.

 Please wait while we load your content...
Please wait while we load your content...