Robustness of thioamide dimer synthon, carbon bonding and thioamide–thioamide stacking in ferrocene-based thiosemicarbazones†
Abstract
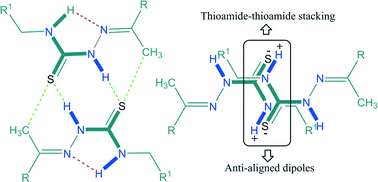
The role of thioureas in crystal engineering as robust supramolecular synthons is now recognized, but their analogs, namely thiosemicarbazones/N-iminothioureas, have not received the attention they deserve. A series of five structurally related ferrocene-based thiosemicarbazones 1–5 have been designed, synthesized and crystallographically characterized in order to investigate the prevalence of the thioamide dimer synthon and carbon bonding. All of the compounds have shown a general preference for the adoption of the cis, trans conformation about the central thiourea moiety which is ideal for the formation of a dimeric hydrogen-bonded R22(8) {⋯H–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S}2 synthon as the building block. Therefore, this dimeric synthon is observed in all of the compounds, with the methyl group particularly set for playing its supportive stabilization role through C–H⋯S and carbon bonding interactions. Carbon bonding has been observed in all of the compounds except compound 2. The centrosymmetrically arranged thioamide protons present in trans conformation through N–H⋯H–C interactions give rise to the formation of tapes of varying topology in all of the compounds except 3, where a staircase arrangement of dimeric molecules is observed. Another notable feature of the crystal packing of 1–5 is the presence of thioamide–thioamide stacking, which has been observed in all five compounds. The prevalence of the thioamide dimer synthon, carbon bonding, and observation of new thioamide–thioamide stacking interactions indicate a much larger role for this class of compounds as a design element in crystal engineering than anticipated so far.
S}2 synthon as the building block. Therefore, this dimeric synthon is observed in all of the compounds, with the methyl group particularly set for playing its supportive stabilization role through C–H⋯S and carbon bonding interactions. Carbon bonding has been observed in all of the compounds except compound 2. The centrosymmetrically arranged thioamide protons present in trans conformation through N–H⋯H–C interactions give rise to the formation of tapes of varying topology in all of the compounds except 3, where a staircase arrangement of dimeric molecules is observed. Another notable feature of the crystal packing of 1–5 is the presence of thioamide–thioamide stacking, which has been observed in all five compounds. The prevalence of the thioamide dimer synthon, carbon bonding, and observation of new thioamide–thioamide stacking interactions indicate a much larger role for this class of compounds as a design element in crystal engineering than anticipated so far.

 Please wait while we load your content...
Please wait while we load your content...