Hydrothermal route to crystallization of FeOOH nanorods via FeCl3·6H2O: effect of Fe3+ concentration on pseudocapacitance of iron-based materials†
Abstract
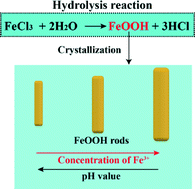
In this work, we studied the crystallization of FeOOH nanorods via hydrolysis of FeCl3·6H2O solution under a low-temperature hydrothermal route (100 °C). The effect of Fe3+ concentration and solution pH on the crystallized morphology and size of FeOOH nanorods was systematically studied based on the chemical reaction and crystallization process. The electrochemical performance of the as-obtained FeOOH materials as supercapacitors is evaluated and the effect of Fe3+ concentration on the pseudocapacitance of iron-based materials is also discussed. FeOOH electrode materials obtained in 0.2 M FeCl3·6H2O solution display the highest specific capacitance of 714.8 F g−1, which is higher than reported values for FeOOH electrode materials. The present work demonstrates a simple hydrolysis route to synthesize high capacitance electrode materials.

 Please wait while we load your content...
Please wait while we load your content...