Construction of Cu(ii), Zn(ii) and Cd(ii) metal–organic frameworks of bis(1,2,4-triazol-4-yl)ethane and benzenetricarboxylate: syntheses, structures and photocatalytic properties†
Abstract
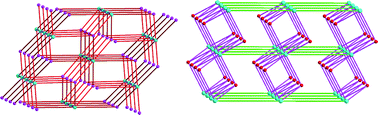
Three coordination polymers {[Cu4(OH)2(btre)(1,2,3-btc)2(H2O)2]·2H2O}n (1), {[Zn4(OH)2(btre)(1,2,3-btc)2(H2O)4]·4H2O}n (2) and {[Cd3(btre)(1,2,3-btc)2(H2O)3]·3H2O}n (3) were synthesized by the hydrothermal method using 1,2-bis(1,2,4-triazol-4-yl)ethane (btre), 1,2,3-benzenetricarboxylate (1,2,3-btc) and metal ions (Cu(II), Zn(II), and Cd(II)). 1 shows a (3,8)-connected 3D metal–organic framework based on the tetranuclear copper(II) cluster [Cu4(μ3-OH)2] with the point symbol of (4·52)3(44·55·612·73·83·9). 2 exhibits a (3,8)-connected 3D metal–organic framework based on the tetranuclear zinc(II) cluster [Zn4(μ3-OH)2] with the point symbol of (42·6)3(46·618·83·10). In 3, each [Cd3(1,2,3-btc)2(H2O)]n chain connects four adjacent identical [Cd3(1,2,3-btc)2(H2O)]n chains through the btre bridges in four different directions, leading to an unusual 3D network. 3 shows a 4-connected 66dia topological net based on the [Cd3(H2O)(COO)4] trimer Cd(II) clusters. The diverse structures demonstrate that the central metal ions play the key role in the assembly of coordination polymers. 1 and 2 show good photocatalytic activity for the degradation of methyl orange. The luminescence of 2 and 3 was investigated.

 Please wait while we load your content...
Please wait while we load your content...