Dimeric packing of molecular clips induced by interactions between π-systems†
Abstract
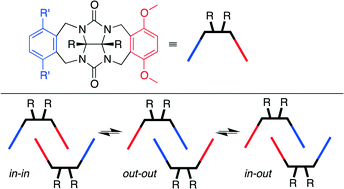
In order to investigate the self-association of asymmetric glycoluril clips, compounds 1–7 with a common p-dimethoxy-o-xylylene ring sidewall were synthesized and their structures were analyzed by X-ray crystallography. As expected, the clip molecules formed dimers promoted by π–π interactions between their aromatic sidewalls. Interestingly, the nature of the substituents on the differentially substituted sidewall caused appreciable differences in the observed dimerization motifs in the crystalline state. For example, 1 and 2 adopted the out–out dimeric motif with its diaromatic-vinyl-o-xylylene rings bound in the cleft of the neighboring molecular clip by π–π stacking interactions. In contrast, compounds 3–7 adopted the in–in dimeric motif in the solid state, in which the p-dimethoxy-o-xylylene rings were sandwiched by the adjacent clip driven by π–π and OCH3⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C H-bonding interactions. X-ray crystallographic analysis of compounds 1–7 indicates that the conformational preferences induced by the linking group between the o-xylylene sidewalls and the terminal aromatic rings plays a critical role in determining which mode of dimerization (in–in versus out–out) and three dimensional packing predominates. The ability to control the selective dimerization of asymmetric glycoluril derived molecular clips via π–π interactions promises to expand the use of these building blocks to design complex and functional solid state architectures.
C H-bonding interactions. X-ray crystallographic analysis of compounds 1–7 indicates that the conformational preferences induced by the linking group between the o-xylylene sidewalls and the terminal aromatic rings plays a critical role in determining which mode of dimerization (in–in versus out–out) and three dimensional packing predominates. The ability to control the selective dimerization of asymmetric glycoluril derived molecular clips via π–π interactions promises to expand the use of these building blocks to design complex and functional solid state architectures.

 Please wait while we load your content...
Please wait while we load your content...