Faceted Cu2O structures with enhanced Li-ion battery anode performances†
Abstract
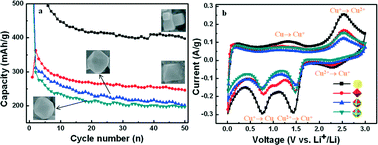
Micro- and nanocrystals with different facets may exhibit different chemical activities that are of great importance in practical applications. The electrochemical performances of Cu2O anodes with different crystal planes are compared. This work comprehensively investigates the effect of the crystal plane on the electrochemical performance of Cu2O. Firstly, Cu2O polyhedra with different morphologies, i.e., cube, octahedron and truncated octahedron, with different degrees of exposed {110} facets, were synthesized. Cu2O cubes show the highest capacity among these Cu2O polyhedra. The present results prove that the {100} facets of Cu2O display high electroactivities toward redox reactions. The electroactivity sequence of the crystal planes is {100} > {111} > {110}. These results confirm that the additional capacity of Cu2O is due to the generation of CuO, which can conduct a two-electron redox reaction. The anode performances of Cu2O polyhedra are dependent on planes, and this can provide a novel route to design high performance electrode materials for lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...