Hydrothermal synthesis and formation mechanism of photocatalytically active SrTiO3 nanocrystals using anatase TiO2 with different facets as a precursor†
Abstract
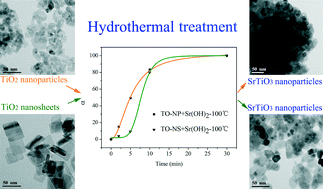
Strontium titanate (SrTiO3) nanocrystals have been synthesized by a hydrothermal reaction route using anatase TiO2 nanosheets with dominant {001} facets and commercial anatase TiO2 nanoparticles as precursors. Time-resolved quenching experiments showed that the speed of hydrothermal formation of SrTiO3 was relatively fast (in minutes). The initial particle morphology and exposed crystal facets of anatase TiO2 precursor cannot be retained in the final SrTiO3 products. SrTiO3 nuclei are formed by the reaction between Ti(OH)x4−x and Sr ions at the TiO2 solid/liquid interface. As surface adsorbed F− is necessary to stabilize the {001} facets of anatase TiO2, the hydrothermal crystallization of SrTiO3 from TiO2 with {001} facets not only exhibits a slower reaction rate but also a higher activation energy. Moreover, the photoelectrochemical and photocatalytic activity studies indicated that the SrTiO3 products obtained from TiO2 with {001} facets showed enhanced photocurrent density and photocatalytic hydrogen production activity. The present study sheds some light on the understanding of the crystal facets of the precursor on the hydrothermal formation process as well as the photocatalytic activities of the corresponding products.


 Please wait while we load your content...
Please wait while we load your content...