Sequential Mukaiyama–Michael reaction induced by carbon acids†
Abstract
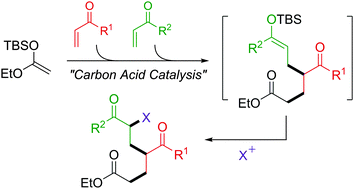
In the presence of a strong carbon acid, the sequential Mukaiyama–Michael reaction using two different Michael acceptors proceeded and the reaction of ketene silyl acetal derived from EtOAc with α-pyrones as primal acceptors yielded the corresponding cyclic ketene silyl acetals, which were reactive enough to undergo the following reaction with second acceptors.

 Please wait while we load your content...
Please wait while we load your content...