Triazole–gold promoted intermolecular propargyl alcohol addition to alkyne: the reaction cascade toward substituted allenes†
Abstract
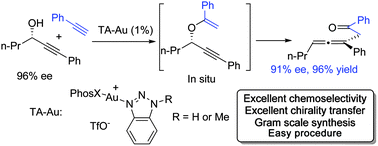
Gold-catalyzed intermolecular propargyl alcohol addition to alkyne was achieved. The reaction of propargyl alcohol with a typical gold catalyst gave the hydration product almost exclusively. The triazole gold catalyst (TA–Au) successfully prevented the hydration, giving the vinyl ether followed by a 3,3-rearrangement. Synthetically useful substituted allenes were formed with high efficiency, substrate tolerability and chemoselectivity. Also, more than 95% chirality transfer from propargyl alcohol to allene was obtained.

 Please wait while we load your content...
Please wait while we load your content...