Alkynol natural products target ALDH2 in cancer cells by irreversible binding to the active site†
Abstract
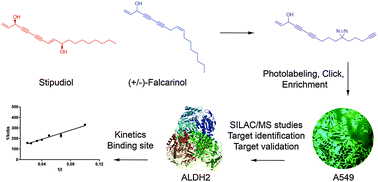
Falcarinol and stipudiol are natural products with potent anti-cancer activity found in several vegetables. Here, we use a chemical proteomic strategy to identify ALDH2 as a molecular target of falcarinol in cancer cells and confirm enzyme inhibition via covalent alkylation of the active site. Furthermore, the synthesis of stipudiol led to the observation that ALDH2 exhibits preference for alkynol-based binders. Inhibition of ALDH2 impairs detoxification of reactive aldehydes and limits oxidative stress response, two crucial pathways for cellular viability.

 Please wait while we load your content...
Please wait while we load your content...