Carbene-derived α-acyl formamidinium cations: organic molecules with readily tunable multiple redox processes†
Abstract
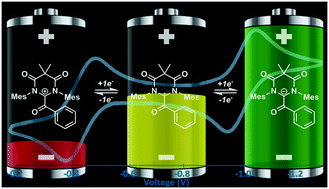
A series of α-acyl formamidinium ions and their corresponding 1-electron reduced neutral radicals were synthesized, and their electrochemical properties were evaluated. These cations exhibit multi-electron redox processes that are highly electrochemically reversible at rapid scan rates (100 mV s−1), and the redox potentials were readily tailored by up to ∼1.0 V, making them ideal candidates for organic radical-based charge storage materials.
- This article is part of the themed collection: 2016 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...