An asymmetric allylic alkylation reaction of 3-alkylidene oxindoles†
Abstract
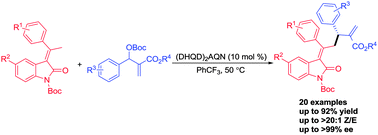
An efficient asymmetric allylic alkylation reaction with respect to 3-alkylidene oxindoles and racemic Morita–Baylis–Hillman carbonate has been achieved by using a chiral biscinchona alkaloid catalyst, which delivers the γ-substituted alkylideneoxindoles with a chiral tertiary center in moderate to good yields (up to 92%) and very good enantioselectivities (up to >99% ee).

 Please wait while we load your content...
Please wait while we load your content...