Spontaneous formation and amplification of an enantioenriched α-amino nitrile: a chiral precursor for Strecker amino acid synthesis†
Abstract
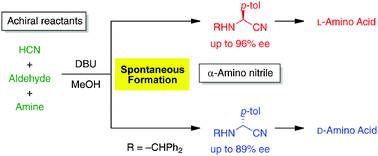
Without the addition of any chiral substances, the spontaneous formation of an enantioenriched α-amino nitrile (up to 96% ee), which is a chiral precursor for Strecker amino acid synthesis, has been achieved in combination with conglomerate formation. The frequency of the formation of enantiomorphs exhibits an approximate stochastic distribution, i.e., L-form occurred 21 times and D-form occurred 22 times, which fulfils the conditions necessary for spontaneous absolute asymmetric synthesis.

 Please wait while we load your content...
Please wait while we load your content...