O-Transfer-facilitated cyclizations of propargylamides with TMSN3: selective synthesis of tetrazoles and dihydroimidazoles†
Abstract
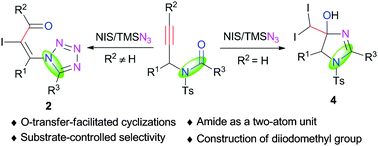
An unprecedented formal [3+2] annulation of propargylamides with TMSN3 to deliver functionalized tetrazoles is developed. Oxygen-atom transfer (OAT) from the amide group to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond was realized via a NIS-triggered-cyclization/ring-opening cascade pathway. The OAT process enables the amide to serve as a two-atom unit in the reactions. Notably, in situ umpolung of azide occurred when terminal propargylamides were employed in this reaction, providing an array of diiodomethylated dihydroimidazoles.
C bond was realized via a NIS-triggered-cyclization/ring-opening cascade pathway. The OAT process enables the amide to serve as a two-atom unit in the reactions. Notably, in situ umpolung of azide occurred when terminal propargylamides were employed in this reaction, providing an array of diiodomethylated dihydroimidazoles.

 Please wait while we load your content...
Please wait while we load your content...