Enhancing the efficiency of sortase–mediated ligations through nickel–peptide complex formation†
Abstract
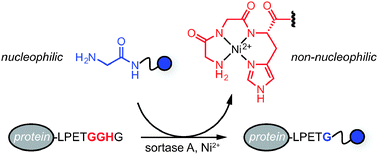
A modified sortase A recognition motif containing a masked Ni2+-binding peptide was employed to boost the efficiency of sortase-catalyzed ligation reactions. Deactivation of the Ni2+-binding peptide using a Ni2+ additive improved reaction performance at low to equimolar ratios of the glycine amine nucleophile and sortase substrate. The success of this approach was demonstrated with both peptide and protein substrates.

 Please wait while we load your content...
Please wait while we load your content...