Multivalent thioglycopeptoids via photoclick chemistry: potent affinities towards LecA and BC2L-A lectins†
Abstract
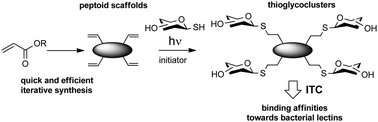
Solution-phase synthesis of linear and cyclic β- and α,β-peptoids was coupled to photo-induced thiol–ene coupling reaction to readily access multivalent thioglycoclusters. A tetrameric cyclic β-peptoid scaffold displaying 1-thio-β-D-galactose or 1-thio-α-D-mannose has revealed by ITC experiments efficient binding potency for bacterial lectins LecA and BC2L-A, respectively.

 Please wait while we load your content...
Please wait while we load your content...