A ring to rule them all: a cyclic ketene acetal comonomer controls the nitroxide-mediated polymerization of methacrylates and confers tunable degradability†
Abstract
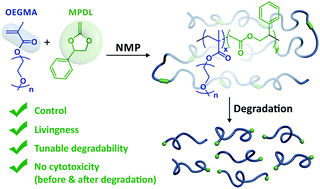
2-Methylene-4-phenyl-1,3-dioxolane (MPDL) was successfully used as a controlling comonomer in NMP with oligo(ethylene glycol) methyl ether methacrylate (MeOEGMA) to prepare well-defined and degradable PEG-based P(MeOEGMA-co-MPDL) copolymers. The level of ester group incorporation is controlled, leading to reductions in molecular weight of up to 95% on hydrolysis. Neither the polymer nor its degradation products displayed cytoxicity. The method was also successfully applied to methyl methacrylate.

 Please wait while we load your content...
Please wait while we load your content...