Catalytic amidation of unactivated ester derivatives mediated by trifluoroethanol†
Abstract
A catalytic amidation method has been developed, employing 2,2,2-trifluoroethanol to facilitate condensation of unactivated esters and amines, enabling the synthesis of a range of amide products in good to excellent yields. Mechanistic studies indicate the reaction proceeds through a trifluoroethanol-derived active ester intermediate.
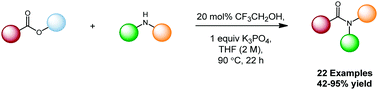

 Please wait while we load your content...
Please wait while we load your content...