A concise formal total synthesis of lactimidomycin†
Abstract
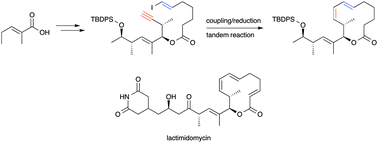
A formal total synthesis of the cytotoxic natural product lactimidomycin has been achieved in nine steps from (E)-2-methyl-2-pentenoic acid. The 12-membered lactone was efficiently formed via a copper-catalyzed ene–yne coupling/alkyne reduction tandem reaction.

 Please wait while we load your content...
Please wait while we load your content...