Intramolecular thiol–yne cyclisation as a novel strategy for thioglycal synthesis†
Abstract
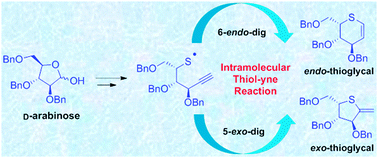
A novel intramolecular thiol–yne cyclisation strategy has been developed for the synthesis of thioglycals. Both ionic and radical mediated cyclisation pathways have been investigated for D- and L-sugars. The ionic cyclisation provides exclusive access to 5-exo products directly from the thioesters whereas the radical cyclisation provides access to both 5-exo and 6-endo products upon photochemical irradiation of the free thiols. These are the first examples of intramolecular thiol–yne cyclisation reactions applied to thiosugar synthesis.

 Please wait while we load your content...
Please wait while we load your content...