Exact helical polymer synthesis by a two-point-covalent-linking protocol between C2-chiral spirobifluorene and C2- or Cs-symmetric anthraquinone monomers†
Abstract
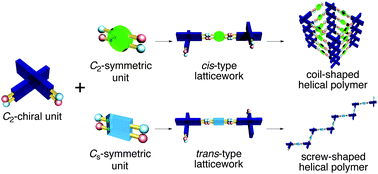
Two types of one-handed exact helical polymers, coil- and screw-shaped polymers, were synthesized by the two-point-covalent-linking protocol using C2-chiral spirobifluorene (SBF) and C2- or Cs-symmetric anthraquinone spacers. Central to this protocol is a new aromatic ring-forming reaction based on the stepwise reductive cyclization of bis(aryloxy group)-substituted anthraquinone derivatives. The helical structures of the polymers annulated by aromatic skeletons exhibited high thermal stability attributed to the rigid C2-chiral SBF units and the covalently two-point-connected structure.

 Please wait while we load your content...
Please wait while we load your content...