Exploiting amphiphilicity: facile metal free access to thianthrenes and related sulphur heterocycles†
Abstract
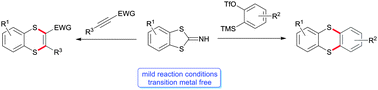
Benzodithioloimines are reacted with arynes or alkynes substituted with electron-withdrawing groups to afford the corresponding thianthrene or benzo[b][1,4]dithiine derivatives. The transformation takes place under mild reaction conditions without any transition metal. Furthermore, the reaction mode could be expanded to 2-thiocyanatopyrroles yielding pyrrolothiazoles.

 Please wait while we load your content...
Please wait while we load your content...