Expanding the scope of strained-alkyne chemistry: a protection–deprotection strategy via the formation of a dicobalt–hexacarbonyl complex†
Abstract
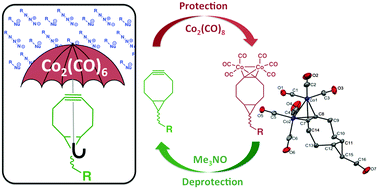
A protection–deprotection strategy for strained alkynes used for bioorthogonal chemistry is reported. A strained alkyne can be protected with dicobalt–octacarbonyl and we demonstrate for the first time that a strained alkyne can be re-formed and isolated under mild reaction conditions for further bioorthogonal reactivity. The protection–deprotection strategy herein reported will expand the versatility of strained alkynes for the preparation of substrates in chemical biology and materials applications.


 Please wait while we load your content...
Please wait while we load your content...