Passerini/Tsuji–Trost strategies towards achieving lactams and cyclopentane derivatives†
Abstract
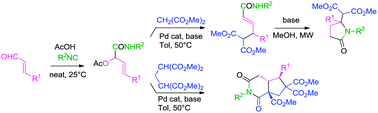
The Passerini reaction of α,β-unsaturated aldehydes affords suitable substrates for the Tsuji–Trost reaction with various carbon based-nucleophiles. The resulting α,β-unsaturated amides may be cyclized to lactams or converted into cyclopentane derivatives if bis-nucleophiles are used in the Tsuji–Trost step.

 Please wait while we load your content...
Please wait while we load your content...