Regioselectivity in the Au-catalyzed hydration and hydroalkoxylation of alkynes
Abstract
Over the past decade and a half, homogenous gold catalysis has emerged as a diverse and rich field of research resulting in the continuous development of new methods for organic synthesis. The activation of alkynes towards nucleophilic attack by AuI and AuIII complexes is a well-established mode of reactivity and the gold-catalyzed hydration and hydroalkoxylation of alkynes are two of the more well-explored reaction pathways. Although these classes of reactions have seen continuous development since their initial reports, achieving regioselectivity persists as one of the most challenging issues for this chemistry. This article aims to draw attention to the general problem of regioselectivity in these reactions. A select set of examples is presented to highlight the challenges and survey some of the strategies employed to address this problem.
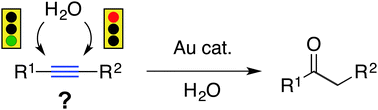

 Please wait while we load your content...
Please wait while we load your content...