Amino acids as chiral anionic ligands for ruthenium based asymmetric olefin metathesis†
Abstract
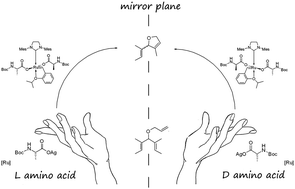
Several amino acid ligands were introduced into the Hoveyda–Grubbs 2nd generation complex by a facile anionic ligand exchange. The chiral pre-catalysts obtained displayed enantioselectivity in asymmetric ring-closing and ring-opening cross-metathesis reactions. Reduction of the lability of the carboxylate ligands was found to be cardinal for improving the observed enantiomeric product enrichment.

 Please wait while we load your content...
Please wait while we load your content...