Efficient electrochemiluminescence of a readily accessible boron difluoride formazanate dye†
Abstract
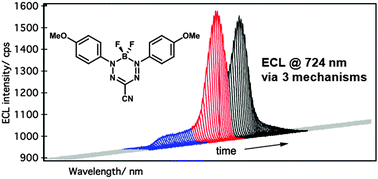
Electrochemiluminescence (ECL) of a boron difluoride formazanate dye was investigated in the presence of tri-n-propylamine as a reductive co-reactant. The ECL mechanism was studied using ECL-voltage curves, spooling ECL, and accumulative ECL spectroscopy. The ECL occurs at 724 nm by three distinct, voltage-dependent mechanisms of light emission.

 Please wait while we load your content...
Please wait while we load your content...